Grading accelerated approval oncology drugs
December 4, 2025Evolent oncology experts have devised a novel metric to help guide decisions about which regimens to include on clinical pathways.
If the FDA’s Accelerated Approval Program were an academic course, it would be graded pass-fail. Some therapies ace the course, while others squeak by, but in the end they all earn the same credit — permission to market a drug.
Still, wouldn’t it be helpful to know which ones are most likely to graduate with full FDA approval and which are at highest risk of dropping out? Of 155 oncology indications receiving accelerated approval status in the last 10 years, 15% have been withdrawn after failing to show clinical benefit in confirmatory trials, and another 41% are still pending approval.
The Clinical Benefit Index (CBI), a new metric designed by a team of Evolent oncology experts to grade the real-world benefit of accelerated approval drugs, aims to address the knowledge gap. While Evolent has been evaluating accelerated approval oncology regimens for years to decide which ones belong on our high value clinical pathways, the CBI is a new way to objectively score and compare regimens. Evolent’s abstract on the Clinical Benefit Index received the Most Innovative Abstract award at the Clinical Pathways Congress in September.
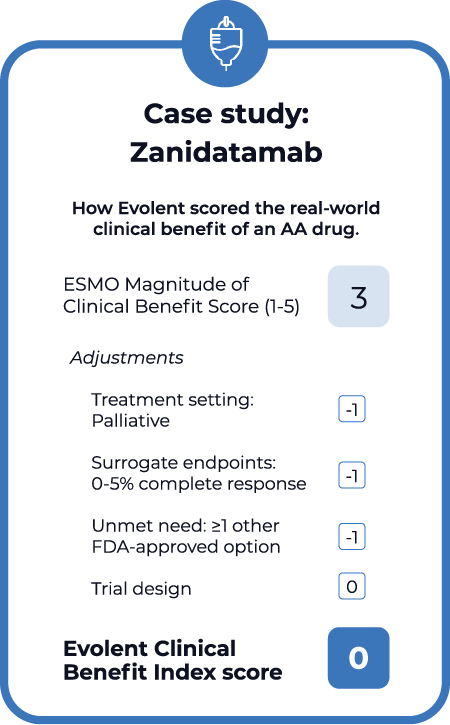
The index builds off the European Society for Medical Oncology’s Magnitude of Clinical Benefit Scale, which grades drugs from 0 to 5 based on early-stage evidence, including long-term survival, toxicity and quality of life. Evolent then adjusts the ESMO score to reflect several real-world considerations:
- Surrogate endpoint quality. Accelerated approvals are typically granted based on surrogate measures rather than the endpoints that matter most, like overall survival. The CBI adjusts the overall score based on the quality of surrogate measures. For example, it adds one point for complete response (CR) rate greater than 10% and subtracts one for CR under 5%.
- Treatment setting. When regimens have curative intent, surrogate endpoints may be stronger predictors of overall survival. Evolent’s CBI adds one point for regimens used in a curative setting and subtracts one for those with palliative intent.
- Unmet need. There may already be other treatment options that offer similar or equal efficacy as a new accelerated approval regimen. Evolent’s CBI adds one point when there are no other options and subtracts one when other options exist.
- Trial design. Evolent also considers how the design of clinical trials, such as the characteristics of the study population, may influence how strongly they predict real-world outcomes. While this is a more subjective component of the CBI, Evolent considers the strength of trial design when assigning a score.
CBI scores will help inform Evolent’s decisions about whether a regimen should be included on our high-value pathways. Those with low scores will be less likely to be included and may wind up on our low-value regimen list. However, there are no strict cutoffs for inclusion or removal.
As the CBI evolves, we also look forward to sharing these scores with our health plan partners and providers to help communicate the benefit of these regimens.